The basic principle of Fuji zirconia oxygen analyzer is: using zirconia as a solid electrolyte, the oxygen concentration on both sides of the electrolyte at high temperature is different to form a concentration battery. The potential generated by the concentration battery is related to the oxygen concentration on both sides, such as oxygen on one side. With a fixed concentration, the oxygen content on the other side can be measured by measuring the output potential.

At high temperature of 600~1200℃, the zirconia material calcined at high temperature has good conductivity to oxygen ions. In the case of unequal oxygen concentrations on both sides of the zirconia tube, the oxygen molecules on the side with the larger concentration combine two electrons on the surface electrode of the zirconia tube to form oxygen ions, and then pass through the oxygen ions in the zirconia material lattice The hole migrates to the side with low oxygen concentration, and when it reaches the low concentration side, it releases two electrons on the side electrode to form oxygen molecules, thus causing charge accumulation on the electrode, and a potential is generated between the two electrodes. The further progress of this migration is hindered until it reaches a dynamic equilibrium state, which forms a concentration cell, which generates a potential related to the difference in oxygen concentration on both sides, called the concentration potential.
In this way, if the zirconia tube is heated to a stable temperature greater than 600°C, and the measured gas and reference gas with the same total pressure flow on both sides of the zirconia, the resulting potential will be the same as the working temperature of the oxide tube and both sides. The oxygen concentration has a fixed relationship. If the reference gas concentration is known, the oxygen concentration of the measured gas can be calculated based on the oxygen potential on both sides of the zirconia tube and the working temperature of the zirconia tube.
In order to correctly measure the oxygen content in flue gas, the following points must be paid attention to when using the Fuji Analyzer:
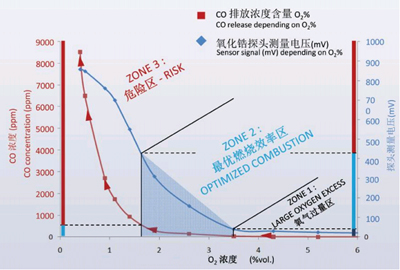
(1) In order to ensure that the output is not affected by temperature, the zirconia tube should work at a constant temperature or additional temperature compensation measures should be added to the instrument circuit.
(2) The pressure of the measured gas and the reference gas should be kept equal during use. Only in this way, the ratio of the partial pressure of oxygen in the two gases can represent the ratio of the percentage volume content of oxygen in the two gases (that is, the oxygen concentration). Because when the pressure is different, if the oxygen concentration is the same, the oxygen partial pressure is also different.
(3) It must be ensured that both the measured gas and the reference gas have a certain flow rate for continuous updating.
